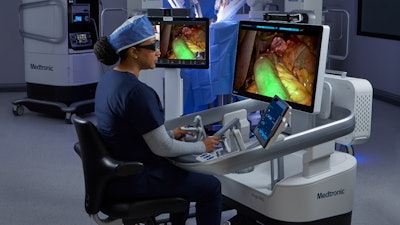
Medtronic has received the CE Mark for the LigaSure RAS vessel-sealing technology, expanding Hugo robotic-assisted surgery (RAS) system capabilities for gynecologic, general, and urologic procedures in Europe.
Powered by the Valleylab FT10 energy platform and for use exclusively with the Hugo RAS system, the LigaSure RAS Maryland instrument is indicated for sealing and cutting vessels, thick tissue, and lymphatics up to and including 7 mm in diameter. The device seals vessels in approximately 2 seconds while minimizing thermal spread to surrounding tissue.
At the Society of Robotic Surgery (SRS) 2025 Annual Meeting, Medtronic will demo telesurgery capabilities on the Hugo RAS system from the IRCAD facility — one of its strategic training partners.
These announcements come amid momentum for the Hugo RAS system, which is now in use across more than 30 countries on five continents.
In the U.S., the company’s submission for a urology indication is under review by the FDA, with an expected U.S. entrance later in the company’s current fiscal year, followed by planned indication expansions into hernia and gynecology.
 Medtronic
Medtronic