BD (Becton, Dickinson and Company) (NYSE: BDX) recently announced the enrollment of the first patient in the investigational device exemption (IDE) study, "Agility," which will assess the safety and effectiveness of the BD Vascular Covered Stent for the treatment of Peripheral Arterial Disease (PAD).
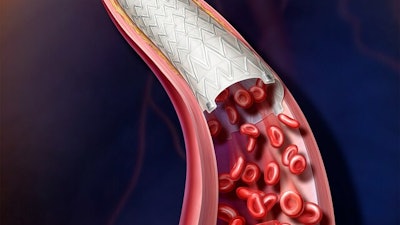
The investigational Vascular Covered Stent is a self-expanding, low profile, polytetrafluoroethylene encapsulated nitinol implant. It is deployed from a delivery system that provides controlled stent release.
"When we're addressing advanced PAD, a self-expanding covered stent can play an important role," said Dr. Sean Lyden, chairman of the Department of Vascular Surgery at Cleveland Clinic and National Principal Investigator of the study. "We need a stent that can track to the lesion, apposes the vessel wall and ultimately provides long-term durability. We're excited to see how this technology performs."
According to BD, the global, prospective, multi-center, single-arm, non-randomized clinical study will include 315 patients at up to 40 clinical study sites across the United States, Europe, Australia and New Zealand. Follow-up for all treated patients will be performed at various points after treatment — starting at one month and ending at 36 months.
PAD affects more than 18 million Americans and more than 236 million people worldwide.1,2 It is a potentially debilitating disease that can lead to increased risk of cardiovascular complications and limb amputation.
A healthy diet, exercise and cessation of smoking can help mitigate the development of PAD, which includes the formation of atherosclerosis and blood clots in arteries in the legs. Minimally invasive techniques using devices such as angioplasty balloons, drug-coated balloons, atherectomy and covered stents can be used to increase blood flow through the diseased areas.
The first patient in the Agility study was enrolled at Trinity Medical Center in Bettendorf, Iowa by Dr. Nicolas Shammas, Interventional Cardiologist, Cardiovascular Medicine, PLLC; Founder and President of the Midwest Cardiovascular Research Foundation, Davenport, Iowa; and Adjunct Professor of Medicine, University of Iowa.






















