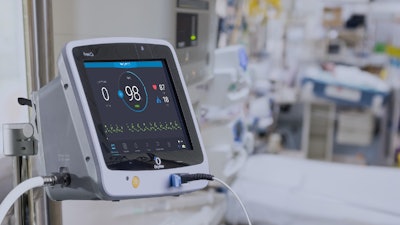
OxyNov has filed a 510(k) submission with the U.S. Food and Drug Administration (FDA) for the FreeO2 device, an oxygen therapy device developed by the company.
This 510(k) submission by OxyNov is an important step in obtaining marketing authorization in the United States for the FreeO2 device.
The application is currently under review by the FDA, and the process is expected to take several months. While waiting for FDA clearance, OxyNov is continuing its preparations for the launch of the FreeO2 device in the United States.
The commercialization of the FreeO2 device in the U.S. market will help establish OxyNov's reputation among distributors.






















