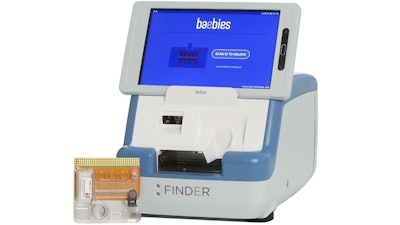
Baebies has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its rapid, point-of-care test for glucose-6-phosphate dehydrogenase (G6PD) deficiency. The test is run on the FINDER platform, which features a toaster-sized instrument and a disposable cartridge.
Using a 50 µL blood sample (one drop of blood), FINDER delivers results approximately 15 minutes after sample introduction. Test results display G6PD enzyme activity in units per gram of hemoglobin and adjusted male median values. The company announced completion of CE Mark for FINDER in December 2019.
The FINDER platform is powered by digital microfluidics (DMF) technology - a method to programming tiny droplets of liquid by electrical control of surface tension on a disposable cartridge. DMF technology eliminates the need for mechanical pumps or valves for liquid handling, reducing the required sample volume and providing fast and reliable diagnostic results.
G6PD deficiency is the first of several assays under development on the FINDER platform. Additional assays under development are focused on addressing unmet needs in hematology and infectious disease through multifunctional syndromic testing, where a seamless combination of multiple types of tests (molecular, chemistry, hematology, immunology, etc) are performed on a single platform to resolve the signs and symptoms associated with a clinical condition.






















