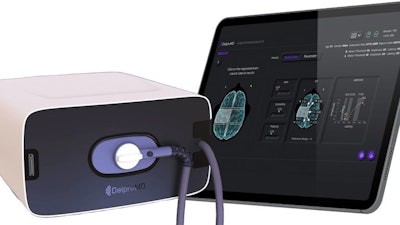
QuantalX, developer of Delphi-MD, an "ECG" for the brain, showcased its brain health technology at the HLTH Las Vegas conference for the first time as part of its U.S. commercialization efforts towards expected FDA breakthrough path clearance.
The Delphi-MD device provides direct brain network visualization, enabling clinical measurements of neuronal functions for the first time in healthcare history, targeting the largest neurological paradigm: "brain abnormalities are usually discovered only after patients are experiencing symptoms and this is already late", said Prof. David Tanne, QuantalX's medical director and president of the IL Neurological association.
For clinicians, such a breakthrough technology, accessible at the point of care, dramatically supports early and differential diagnosis through "real life" objective data, leading to informed treatment decisions and interventions, as well as improving brain health outcomes for healthcare providers and payors. For patients, an earlier diagnosis leads to an optimal care pathway, resulting in a positive, long-term effect on quality of life.
35% of the total economic disease burden is attributed to brain disorders, mostly due to repeated hospitalization and rehabilitation, avoidance of unnecessary CT/MRI scans and associated accessibility and cost challenges. Recent estimations* show the financial burden of stroke and dementia care alone to exceed $550B/ year in the US alone. "The clinical breakthrough of Delphi-MD is that it is a direct, functional, objective test for the brain, that does not require patient participation and provides actionable insight to the physician at the point of care," said Dr. Iftach Dolev, CEO and Co-Founder of QuantalX. "Making neuro-diagnosis accessible to everyone is game changing for brain health management"






















