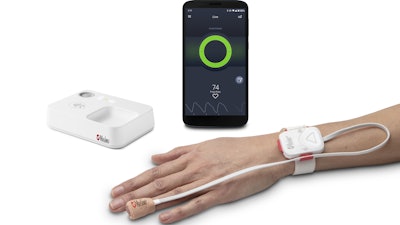
Masimo today announced that Masimo Opioid Halo, an opioid overdose prevention and alert system, has been granted a De Novo by the FDA – making it the first and only FDA-cleared monitoring solution for detecting opioid-induced respiratory depression, the leading cause of death from opioid overdose. With the De Novo, Masimo also becomes the first winner of an FDA Opioid Innovation Challenge to have an authorized solution designed to help solve the U.S. opioid crisis.
The De Novo authorizes Opioid Halo to be made available over the counter (OTC) without a prescription, for use on adults and children age 15 and up, and an Rx version for use by prescription from a healthcare provider. Opioid Halo advances the forefront of continuous monitoring through its unique Opioid Halo engine, an advanced pattern recognition algorithm which helps detect and quantify the risk of severe opioid-induced respiratory depression. Combined with its innovative distributed architecture, Opioid Halo helps to manage and send escalating alarms to family members, friends, and caregivers, notifying them that help may be needed due to an opioid overdose – including triggering an automatic wellness call, which may lead to EMS being dispatched.
Masimo Opioid Halo is designed to help family and friends identify the symptoms of an opioid overdose by detecting physiological markers present during opioid-induced respiratory depression and ideally, helping them know when it’s time to intervene – for example, by administering a potentially life-saving dose of naloxone. Opioid Halo can be used at home or in the hospital or another care setting, by patients prescribed opioids after surgery or managing a chronic or prolonged condition, as well as people suffering from opioid use disorder.





















