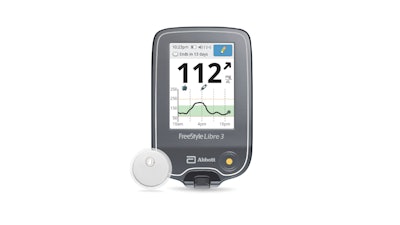
Abbott today announced that the U.S. Food and Drug Administration (FDA) has cleared a reader for its FreeStyle Libre 3 integrated continuous glucose monitoring (iCGM) system, which features the world's smallest, thinnest and most discreet glucose sensor. With the FDA's clearance of a standalone reader, Abbott is working to get the FreeStyle Libre 3 system added to Medicare's list of covered systems as soon as possible.
The FreeStyle Libre 3 reader is a small handheld device that displays real-time glucose readings directly from a small sensor worn on the back of a person's upper arm, allowing them to manage their diabetes quickly and easily by viewing their glucose readings on a large, bright and easy-to-see screen.
People who use the FreeStyle Libre 3 system will still have the option to use the current FreeStyle Libre 3 smartphone apps.
The reader uses a rechargeable lithium-ion battery, which is commonly found in many other electronic devices like mobile phones. The user manual for the FreeStyle Libre 3 reader provides details on how to safely store, charge and use the device, including always using the Abbott-provided USB cable and power adapter.
The FreeStyle Libre portfolio is the number one sensor-based glucose monitoring system in the world, having changed the lives of 4.5 million people across more than 60 countries by providing breakthrough technology that is accessible and affordable.





















