Mosie Baby, an at-home fertility care company, has secured FDA Class II clearance for its Mosie Baby Kit, which the company said makes it the first and only FDA-cleared over-the-counter kit for use in intravaginal insemination (IVI). The kit was created to support those who are unable to conceive with intercourse or for whom intercourse is not an option. Following its FDA 510k Class II clearance, Mosie Baby looks forward to expanding access to its Mosie Baby Kit which was determined to be "substantially equivalent" to a predicate device used in clinically administered intrauterine insemination (IUI).
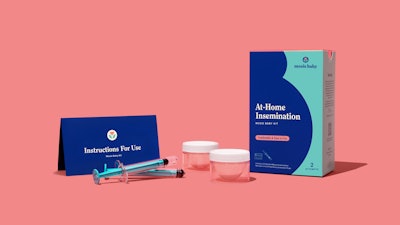
Designed to be used with either a fresh or cryogenically frozen donor semen sample, each Mosie Baby Kit includes two patented syringes, which are specifically designed for at-home insemination, and two proprietary collection cups that were created for semen collection. Mosie Baby's syringe features a patented barrel-free tip and slit opening which optimizes transfer while minimizing waste, while its collection cup has a proprietary design that supports maximum collection. As part of Mosie Baby's FDA Class II clearance, the Mosie Baby Kit was put through rigorous clinical and technical testing, including Human Sperm Survival Assay, vaginal irritation testing, and biocompatibility testing. Through these tests, Mosie Baby validated that the device is non-cytotoxic, non-irritating, non-sensitizing, and free from microbial contamination.
The Mosie Baby At-Home Insemination Kit (MSRP: $129.99) is available for purchase at mosiebaby.com, CVS.com or at select CVS stores nationwide, Walmart.com and optumstore.com. The kit will launch with additional retailers and healthcare partners in 2024.




















