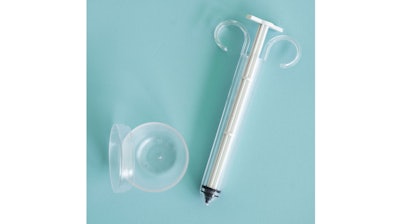
PherDal Fertility Science, creators of a sterile, at-home insemination kit, is now accepting pre-orders of its intravaginal insemination (IVI) device, following FDA class II clearance. The company said the PherDal Kit is the first and only to combine the sterile environment of the fertility clinic with the at-home capabilities of insemination.
Each PherDal Kit includes three sterile syringes and three sterile collection cups to make at-home insemination as sterile, safe and accessible as possible. Unique to the PherDal Kit, the sterile syringe is intentionally designed to deliver sperm at the opening of the cervix, while bypassing bacterial or anatomical interference, which studies have linked to infertility.
Now, PherDal is bridging the gap between traditional conception and more invasive options with a sterile at-home insemination option for individuals who have been unable to conceive through intercourse or have chosen not to conceive through intercourse.
While PherDal started as the founder’s story, the proof of concept kit launched in 2021, selling out 200 units in 90 days. From those 200 kits, the PherDal community celebrated 34 pregnancies, including the addition of the first PherDal siblings this year.
In 2022, PherDal crowdfunded over $630,000 to pursue FDA clearance. In 2023, Dr. Hintzsche was a top 5 finalist at the SXSW Pitch competition, a top 3 finalist for the Insight Innovation Labs Startup Competition, and featured innovator at LSI’s Emerging MedTech Conference. PherDal has been selected for the Femovate 2023-2024 cohort of global FemTech startups.






















