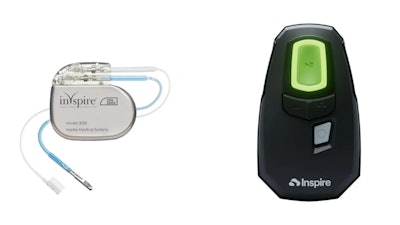
Inspire Medical Systems, a medical technology company focused on the development and commercialization of minimally invasive solutions for patients with obstructive sleep apnea (OSA), announced the FDA approval of the Inspire V therapy system which includes the next generation neurostimulator and the associated Bluetooth patient remote and physician programmer.
Inspire therapy is a mask-free solution that includes the Inspire implant, remote and app, it lets users control their OSA treatment with a handheld device.
“We are thrilled to announce the FDA approval of our next generation Inspire neurostimulation system,” said Tim Herbert, Chairman and Chief Executive Officer of Inspire Medical Systems. “The FDA approval marks a key milestone for the future of Inspire therapy and reinforces the many years of hard work by our team members.”
The company’s focus remains on operational readiness, which includes product manufacturing and establishing inventory levels to support a full commercial launch in the United States. The company continues to target a soft launch in late 2024 and a full launch in 2025.






















