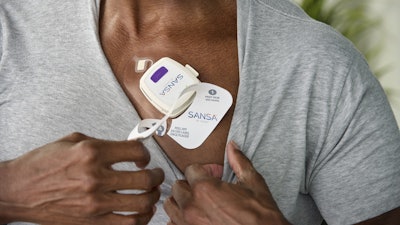
Huxley Medica has received 510(k) clearance from the FDA for its chest-worn, sleep apnea diagnostic patch, SANSA. With this clearance, Huxley Medical can move forward to market its device.
The patch is an at-home, single-point-of-skin-contact diagnostic device. Unlike current patches, rings, watches, or finger probes, SANSA does not require additional attachments, wires, belts, or hoses. Its patented combination of sensors and materials measures eight physiological channels including blood oxygen saturation, EKG-derived heart rate, respiratory effort, chest movement, sleep staging, snoring, body position, and actigraphy.
The SANSA platform has already undergone a clinical trial involving 533 patients across seven U.S. sites, including at the University of Pennsylvania, the University of Michigan, Emory University, and Atrium Health Wake Forest Baptist. SANSA has demonstrated its efficacy in measurement reliability and performance for use in diagnosing mild, moderate, and severe sleep apnea.
The clinical trial provided robust data supporting SANSA's safety and effectiveness, including:
- high accuracy, sensitivity, and specificity in sleep apnea detection compared to in-lab sleep tests;
- improved patient compliance and comfort due to its non-invasive nature;
- excellent performance on all skin tones for diverse patient management, which had been a challenge in the past.






















