Womed, a uterine health company developing treatments to free women from uterine pathologies, today announced it has signed licensing agreements with Kebomed Europe and Saesco Medical to distribute its first medical device, Womed Leaf, in Europe.
Intrauterine Adhesions (IUA), which refer to the pathological binding of the uterine walls, are caused by scarring of the uterus after procedures such as dilation and curettage or fibroid removal, and can occur in 20% to 45% of those procedures. IUAs are a major cause of infertility, recurrent miscarriages and pain.
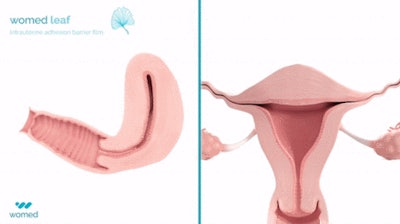
Womed said the Leaf is the first and only physical barrier to protect against IUA. The device consists of a soft, flexible film made from Womed's polymer, which is inserted like an IUD at the end of a uterine procedure. It expands within the cavity, preventing contact between the uterine walls for one week, and is then naturally and painlessly discharged. Womed Leaf was proven to be highly effective in the PREG2 randomized clinical study that enrolled 160 patients with severe or moderate IUA.
“These partnerships mark the first step of a global launch that will make Womed Leaf the new gold standard in intrauterine adhesion prevention and treatment,” said Gonzague Issenmann, Co-founder and CEO of Womed. “Kebomed Europe and Saesco Medical’s established sales networks and expertise in women’s health will help European women and their doctors to preserve or recover their fertility."
"Womed Leaf addresses a significant unmet need in women's health and we are excited to partner with Womed to bring it to our customers," said Lars Melbye, Managing Director of Kebomed Europe. Jordi Fluvia, CEO of Saesco Medical, added: "We are committed to offering the latest advances in medical technologies, and Womed is bringing a true innovation in an indication that has been sorely lacking in meaningful improvements for the last two decades.”
Kebomed Europe will have the responsibility of selling the device in France, Germany, Sweden, Denmark, Norway, Finland, Austria and Switzerland, while Saesco Medical will cover Spain, Italy, Portugal, Belgium, the Netherlands and Luxembourg.






















