Neuros Medical, the maker of the Altius Direct Electrical Nerve Stimulation System, an FDA-approved, non-opioid treatment for chronic post-amputation pain, closed an oversubscribed $56 million Series D financing round. The funding will support U.S. commercialization and further development of Altius Direct.
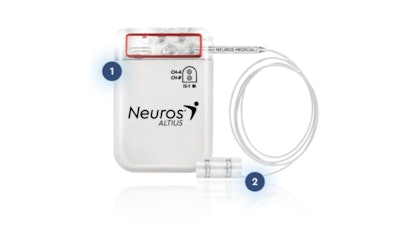
The Altius System is a patient-controlled, on-demand system that uses Neuros' patented technology to address the underlying cause of post-amputation pain by inhibiting pain signal transmission from the damaged peripheral nerves near the site of amputation to the central nervous system. The system consists of a nerve cuff electrode placed around an affected nerve and an implantable pulse generator (IPG). Patients initiate an on-demand 30-minute treatment session as needed for targeted pain relief.
The Altius System is FDA-approved and is indicated as an aid in the management of chronic intractable phantom and residual lower limb post-amputation pain in adult amputees. The Altius System is the only FDA-approved device that provides amputees with patient-controlled, on-demand relief by targeting the nerve pain directly.






















