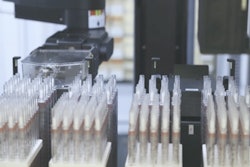
Roche received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the cobas pure integrated solutions, the next generation of innovation in the cobas family of Serum Work Area solutions.
The new compact and modular solution combines three technologies – clinical chemistry, immunoassay and Ion-Selective Electrode (ISE) diagnostic testing – on a single platform, helping to optimize space and resources for low- to mid-volume labs to support the delivery of the best patient care.
Built on the latest technology, cobas pure integrated solutions focuses on the automation of manual tasks, reducing the hands-on maintenance time of technicians to just five minutes per day. This can help to improve the productivity of lab personnel while also supporting fast delivery of patient results and clinical decision-making.
Laboratory testing provides essential information for the prevention, diagnosis, treatment and management of disease. This helps patients receive the best care and highest quality outcomes. The cobas pure system delivers test results in short and predictable turnaround times using low-volume patient samples.
With a footprint of about 21 square feet, cobas pure integrated solutions is up to 30% smaller than previous generation systems. It is able to perform up to 870 tests per hour with access to Roche’s full clinical chemistry and immunochemistry assay menu.
Within the first year after launch, the menu will include more than 186 diagnostic tests across a wide-range of disease areas such as infectious diseases, oncology and cardiology. The system will enable low- to mid-volume labs to make better use of their space and expand their offering of high medical value tests for the benefit of patients.
Furthermore, to ensure effective and efficient work within healthcare networks, the cobas pure system operates seamlessly with Roche’s cobas pro integrated solutions for mid- to high-volume labs.
Standardization helps labs do more work on fewer instruments, deliver consistent results and facilitate flexible staffing strategies through features including consolidated workflows, shared reagent packs, standard reference ranges and common system interfaces.
The cobas pure integrated solutions launched in countries accepting the CE mark in March 2021.