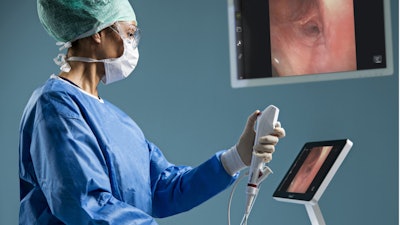
Ambu said its aScopeTM 5 Broncho, a family of single-use, sterile bronchoscopes, has received 510(k) regulatory clearance from the U.S. Food and Drug Administration (FDA).
Ambu announced European regulatory clearance in May 2022 and will now proceed with commercialization of the aScope 5 Broncho and the full high definition Ambu aBoxTM 2 processing unit in Europe as well as in the USA.
With the aScope 5 Broncho, Ambu now leads the entry of single-use endoscopes in the bronchoscopy suite, a market segment known for its considerably complex medical procedures — procedures that require scopes of high-performance image quality and handling. To enter this market, the aScope 5 Broncho family has advanced imaging and design features, including a new high-resolution camera chip, which, in combination with the aBox 2, delivers superior image quality.