A new study from PICC Excellence shows that standardizing ultrasound-guided PIV insertions in a hospital setting using an innovative sterile barrier dressing reduced costs by 73% and staff time for insertions by 50%, while also improving both patient and nursing staff satisfaction.
The results were presented in a poster by Nancy Moureau, RN, PhD, CRNI, CPUI, VA-BC, and Pam Pressnall, RN, VA-BC, at the recent meeting of the Association for Vascular Access (AVA), September 30 - October 3, 2022, in Minneapolis.
“Our study shows that it is possible to improve outcomes and procedures for ultrasound-guided PIV insertions while also significantly cutting costs and staff time and reducing waste,” said Dr. Moureau, CEO of PICC Excellence and an internationally recognized expert and consultant in vascular access. “These results suggest that hospitals should consider more widespread use of the standardized UltraDrape dressing procedure.”
Study Background
The study was done at a medium-sized regional Florida hospital with 245 beds and an emergency department that sees about 160 patients per day. A five-member vascular access team manages about 90 ultrasound-guided insertions per month.
As part of a quality improvement initiative, researchers evaluated existing procedures for ultrasound-guided PIV insertions. They found significant procedural and supply variations among the departments. While the hospital did consistently use a Central Line Dressing Kit for each insertion, researchers observed that inserters wasted some of the more expensive components of the Kit.
In addition, patients reported dissatisfaction because some insertions required multiple attempts.
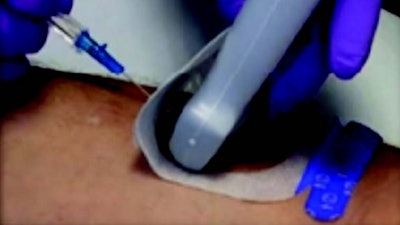
To test possible solutions to these problems, the hospital switched to a standardized approach using an intravenous Start Kit with a sterile barrier ultrasound dressing called UltraDrape, (Parker Laboratories, Inc.).
In the standardized procedure, inserters first mark the selected site and apply the folded UltraDrape dressing to the skin. The ultrasound gel is applied to the back side of the dressing for ultrasound probe position and guidance during the insertion. Finally, the gel layer of the dressing is lifted off, and the dressing pulled down over the catheter, creating a sterile transparent dressing cover.
Study Results
When the hospital switched to the standardized procedure, the data collected demonstrated impressive results. The average cost of supplies per insertion dropped from $25.32 to $6.71, which would add up to savings of more than $20,000 per year.
In addition, the time required for each insertion was reduced from 6.51-12.14 minutes to just 3.2-4.25 minutes. Moreover, both patients and staff reported being far happier because of quicker insertions and fewer failed attempts.
“The UltraDrape dressing enables a level of standardization that simplifies the procedure while ensuring that sterile conditions are maintained,” said co-author Pam Pressnall. “This helped us achieve a level of efficiency that allows us to perform more ultrasound-guided patient insertions in fewer attempts, resulting in happier patients and nursing staff.”
UltraDrape Minimizes UGPIV Risks
Dr. Moureau also gave a talk at the 2022 AVA meeting describing the history of—and the rationale for—the development of the UltraDrape dressing. She emphasized that the UltraDrape is simple to use, cost effective and faster than using standard probe covers. Using ultrasound to guide PIV insertions also improves the success rate for insertions, she explained, especially for patients with more co-morbidities requiring special skill with visualization technology.
But the use of ultrasound also raises significant challenges: the presence of both the ultrasound probe and gel on at the sterile insertion site can contribute to contamination. In addition, gel requires difficult clean up, and any gel left on the skin reduces the adherence of the dressing, increasing the chances of dressing failure.
UltraDrape was designed to solve these problems, she explained. It is the first dressing designed specifically for use during ultrasound-guided PIV insertions. The ultrasound gel is applied to a disposable film layer instead of the patient’s skin, resulting in a safer, gel-free insertion. This also eliminates the time-consuming post-procedure clean-up and reduces the risk that inadequate gel removal will lead to dressing failure.
“It is the only dressing that separates the gel from the insertion site and that removes the gel after insertion, while providing sterile probe protection,” she said. “For the estimated 12 million ultrasound-guided PIV insertions done each year in North America, it offers a win-win solution, simplifying and speeding up the procedure while reducing costs and time.”