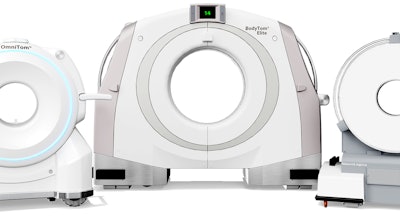
NeuroLogica, a subsidiary of Samsung Electronics, announced that its Elite Mobile Computed Tomography (CT) systems have received the European Union (EU) CE marking via compliance with the new EU Medical Device Regulation (MDR 2017/745).
The assessment and certification completed by the EU’s Notified Body includes the approval of the OmniTom Elite, BodyTom Elite and CereTom Elite mobile CT systems.
The EU MDR (EU Medical Device Regulation 2017/745) replaced the previous EU MDD (Medical Device Directive 93/42/EEC). The updated regulations place a strong emphasis on technical documentation, clinical data and post-market surveillance.
“NeuroLogica already complies with the United States Food and Drug Administration quality system regulations and is certified to ISO 13485 standard. Our products and processes comply with global regulatory requirements allowing design, manufacturing, installation, service and engineering of imaging systems for medical applications,” said Dr. Ninad Gujar, Vice President of Regulatory Affairs, Quality Assurance and Corporate Compliance. “Compliance with EU MDR is an important regulatory milestone that exhibits NeuroLogica’s efforts towards demonstrating conformance to EU regulatory requirements, making mobile CT systems available in the European Economic Area and remaining committed to product quality and safety.”
Currently providing advanced technology to more than 50 countries globally, NeuroLogica has existing partners in Europe working as Economic Operators and an established network of distributors to continue to support customers. The company’s mobile CT product line will continue to be manufactured in Danvers, Massachusetts in the United States, and will be exported to customers in the EU.