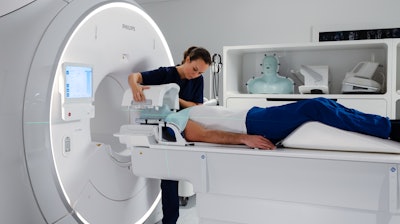
Royal Philips announced two new advances in MR-only workflows to advance head and neck cancer radiotherapy imaging and simulation. The company’s artificial intelligence (AI) enabled MRCAT Head and Neck radiotherapy application, which allows the use of MR as the sole or primary imaging modality for radiotherapy planning in the treatment of soft tissue tumors in the head and neck, along with the brain, pelvis and prostate, has received FDA 510(k) clearance and is commercially available in the U.S.
As a result of a recent development partnership with patient-positioning company MacroMedics, Philips has also announced compatibility of MacroMedics’ latest DSPS (Double Shell Positioning System) Prominent positioning system with Philips MR Head Neck Coil. This unique solution combines the superior soft tissue imaging capabilities and the high-resolution image quality of Philips’ head and neck coil with the comfort-enhancing and positional accuracy and stability features of MacroMedics’ mask. These developments aim to improve the accuracy of radiotherapy planning and simulation to help achieve better patient outcomes, enhance patient comfort, and offer the efficiency benefits of an MR-only workflow.