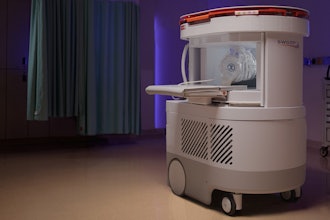
Hyperfine, the medical device company that created the Swoop system, the world’s first FDA-cleared portable magnetic resonance brain imaging system, has announced the commencement of an international, multi-site observational study, ACTION PMR (ACuTe Ischemic strOke detectioN with Portable MR). To support its acute stroke care initiatives, Hyperfine Inc. has formed an advisory board of world-renowned stroke experts.
As a prospective, international, multi-site observational study, ACTION PMR aims to examine the integration of brain imaging with the Swoop system into the stroke diagnosis and treatment workflow. The goal is to use point-of-care brain imaging to identify strokes and viable brain tissue that can be saved.
MRI scans, which are more precise than CT scans, are recommended for diagnosing acute ischemic stroke within twelve hours of symptom onset by the American Academy of Neuroradiology. However, the limited availability of MRI scanners near acute care settings in many hospitals highlights the need for point-of-care MR brain imaging.
Four investigators from leading institutions will lead the study: Dr. W. Taylor Kimberly of Massachusetts General Hospital, Dr. Adnan Siddiqui of the University at Buffalo, Dr. Vivien Lee of the Ohio State University Wexner Medical Center, and Dr. Keith Muir of the University of Glasgow.
Alongside the ACTION PMR study, the newly formed stroke advisory board, comprised of experts in the field, will provide insights and experiences to guide and define the impact the Swoop system can have in the fast-evolving field of acute stroke care.