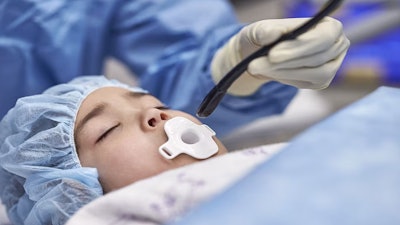
Philips announced that its latest TEE transducer, designed to serve more patients with improved overall comfort, has received FDA 510(k) clearance.
The ‘transesophageal echocardiography’ (TEE) ultrasound transducer helps cardiologists by providing highly detailed images of the heart and its internal structures. In structural heart disease, the quality of a 3D TEE image can help save lives. The clarity and perspective that come with 3D images exceed 2D alternatives. TEE is also a valuable tool for minimally invasive heart surgeries and procedures, transforming the treatment of damaged heart valves and congenital heart defects.
However, there were some patients who still couldn’t benefit from this advanced technology, including pediatric patients as small as 5 kg, adults at risk of complications, as well as complex cases such as ICU patients, where the transducer probe for 3D TEE was too large. Until now. Announced today, Philips’ new X11-4t Mini 3D TEE transducer is shifting that balance and opening 3D TEE imaging to previously unaddressed patients.
Developed to be easily tolerated by patients due to its 35% smaller size and pill-shaped design, Philips’ new X11-4t aims to enhance the patient experience, with 87% of clinical respondents stating they believe the X11-4t may contribute to improved overall patient comfort. Clinical staff can also benefit from a Mini Live 3D TEE solution that allows them to care for a wider range of patients, using the same hand control, procedure navigation techniques and workflow they are familiar with on Philips’ EPIQ cardiac ultrasound systems. As a result, echocardiographers require minimal additional training on this newest transducer.The new transducer is compatible with Philips’ premium cardiology ultrasound portfolio including the EPIQ CVx and EchoNavigator image-guided therapy solution, allowing clinicians to deliver personalized, efficient, and clinically smart cardiac care to help improve outcomes and the patient experience. It also joins a portfolio that includes another significant innovation – VeriSight Pro – the first 3D intracardiac echocardiography catheter to miniaturize the same 3D imaging technology that powers TEE.
More than 100 cardiac measurements are routinely performed as part of every echocardiography exam, taking up 50% of exam time. The new X11-4t Mini 3D transducer reaffirms Philips’ commitment to deliver industry first innovations to help improve productivity and increase clinical decision support, which includes AI-enabled technology and automated tools like the Dynamic Heart Model to aid in procedural guidance. Philips AI-enabled cardiac ultrasound is building on the industry leading AI technology capabilities from DiA Imaging Analysis.