FUJIFILM Healthcare Americas received 510(k) clearance for CAD EYE, the company’s novel AI detection system for endoscopic imaging. CAD EYE enables real-time detection of colonic mucosal lesions such as polyps and adenomas during colonoscopy procedures, supporting endoscopists in their ability to detect and remove pre-cancerous lesions, regardless of size, shape and color.
Consisting of a compatible expansion unit (the Fujifilm EX-1) and endoscopy support software (EW10-EC02), CAD EYE is an evolution of Fujifilm’s ELUXEO Endoscopic Imaging System, featuring AI image processing functionality customized for the integration with the system’s processor and the endoscope. It was developed using deep learning technology in Fujifilm’s Tokyo-based global AI technology center and validated in several studies using histologically confirmed polyps in clinical images obtained through Fujifilm endoscopy systems.
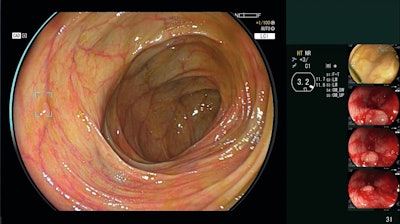
This technology is designed to support detection of lesions that may be easiest to miss, such as flat lesions, those at the corner of the endoscopic view, and multiple lesions present in a single frame. When a suspicious polyp is detected by CAD EYE, the physician is automatically given both visual and auditory alerts: a “Detection Box” appears, a “Visual Assist Circle” illuminates in the area, and an alert is sounded, which can be adjusted per the user’s preferences. The visual cues are overlaid on top of – but do not interfere with – clinical images in the physician’s existing workflow.
CAD EYE can be used with both White Light Imaging as well as Linked Color Imaging (LCI), Fujifilm's enhanced visualization mode that differentiates the red color spectrum to enhance mucosal visualization. LCI is clinically proven to improve visualization, detection, and documentation of colonic polyps. Both CAD EYE and LCI can be launched at the touch of a button, either on the endoscope handle or the processor unit.
CAD EYE will be commercially available this spring 2024 following the completion of a limited market evaluation.