RefleXion Medical, a therapeutic oncology company, today announced the U.S. Food and Drug Administration (FDA) has granted the first marketing clearance for its SCINTIX biology-guided radiotherapy, a cutting-edge treatment applicable for early and late-stage cancers. SCINTIX is the first and only radiotherapy that allows each cancer’s unique biology to autonomously determine where and how much radiation to deliver, second-by-second, during actual treatment delivery.
This expands the RefleXion X1 into the only dual-treatment modality platform that can treat patients with indicated solid tumors of any stage. The SCINTIX biologic modality tracks tumor motion from all types of movement, including expected motion from internal processes such as breathing and digestion or unexpected movement by a patient. The X1 also has a state-of-the-art anatomic modality previously cleared by the FDA for solid tumors located anywhere in the body.
The FDA cleared SCINTIX biology-guided radiotherapy to treat patients with lung and bone tumors. These tumors may arise from primary cancers or from metastatic lesions spread from other cancers in the body. Previously granted Breakthrough Device designation by the FDA for treating lung tumors, the breakthrough nature of SCINTIX technology lies in its ability to detect and then treat multiple moving tumors. Initially cleared for use with the radiopharmaceutical fluorodeoxyglucose F 18—commonly known as FDG—the company plans to adapt SCINTIX therapy to work with the full array of novel radiopharmaceuticals under development for different cancer types.
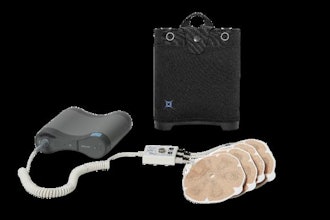
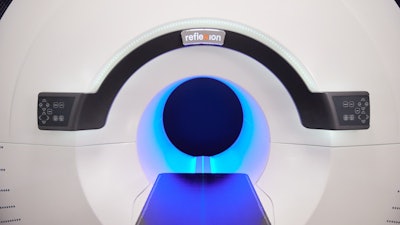
SCINTIX therapy (formerly referred to as BgRT) is delivered through the RefleXion X1 machine, which combines positron emission tomography (PET) with a linear accelerator (LINAC) to deliver a radiation dose that tracks the cancer’s motion. Immediately prior to treatment, the patient is injected with a radiopharmaceutical that interacts with cancer cells to produce signals or emissions. The X1 continuously constructs a map from the detected emissions data that determines where to aim beamlets of radiation. This crosstalk between the tumor and the X1 requires the system to rotate at 60 rpm—making it the first and only radiotherapy machine to spin at this speed.
Combined, tumors in the lung or bone, including those that metastasize from other primary cancers, arise in approximately 430,000 patients. In metastatic cancer, where patients can present with both lung and bone tumors, radiotherapy is often not considered as a treatment option due to the cumbersome clinical workflow and toxicity of treating more than one tumor in a treatment session.