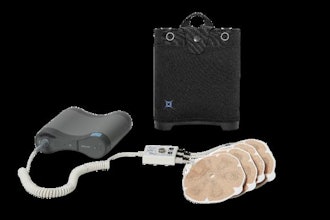
Medtronic said it has received FDA 510(k) clearance for its OsteoCool 2.0 bone tumor ablation system for the treatment of painful bone metastases and benign bone tumors such as osteoid osteoma.
An upgraded design of the OsteoCool radiofrequency ablation system brings several new advantages over the previous system. It allows for the simultaneous use of 4 internally cooled probes, enabling physicians to ablate two vertebral bodies at once or create larger ablation zones in extra-spinal applications. The company said OsteoCool 2.0 is the most powerful bone tumor ablation system on the market, delivering 20W per channel. It also offers the widest selection of probe sizes in the U.S. market with 4 available options.
The OsteoCool platform remains one of the only bone tumor ablation platform with internally-cooled probes on the market, delivering predictable ablations, reduced risk of excess heating around the lesion site, and proven pain relief. The clinically proven technology is backed by OPuS One, the largest study of RF ablation in bone metastases, which demonstrated swift (within 3 days), significant, and sustained (through 12 months) improvements in pain relief for cancer patients.
For patients with metastatic cancer, between 60-80% may develop bone tumors. These are found most frequently among patients with primary malignancies of the breast, prostate, and lung.
Metastatic bone tumor ablation involves a minimally invasive procedure using probes to deliver radiofrequency energy that heats and destroys the tumor while circulating water to cool the probes in close proximity to the active tip, to avoid excess heating.
Medtronic will initiate a limited market release of OsteoCool 2.0 immediately, with a broad U.S. market launch planned for later this calendar year.