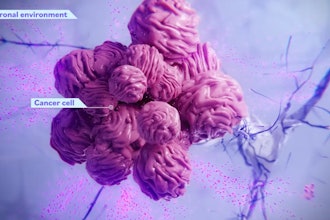
WEST LAFAYETTE, Ind. — Purdue University researchers are developing and validating a patent-pending treatment for incurable glioblastoma brain tumors. Glioblastomas are almost always lethal with a median survival time of 14 months. Traditional methods used against other cancers, like chemotherapy and immunotherapy, are often ineffective on glioblastoma.
Sandro Matosevic, associate professor in the Department of Industrial and Molecular Pharmaceutics in Purdue’s College of Pharmacy, leads a team of researchers that is developing a novel immunotherapy to be used against glioblastoma. Matosevic is also on the faculty of the Purdue Institute for Cancer Research and the Purdue Institute for Drug Discovery.
The Matosevic-led research has been published in the peer-reviewed journal Nature Communications.
The Purdue glioblastoma treatment
Matosevic said traditional cell therapies have almost exclusively been autologous, meaning taken from and returned to the same patient. Blood cells from a patient are engineered to better recognize and bind to proteins on cancer cells, then given back to the same patient to bind to and attack cancer cells. Unfortunately, these therapies have limited to no effect on glioblastoma.
“By contrast, we are developing immunotherapy based on novel, genetically engineered, fully off-the-shelf or allogeneic immune cells. Allogeneic cells are not sourced from the same patient, but rather another source,” Matosevic said. “In our study, we sourced — or rather engineered — cells from induced pluripotent stem cells. So we eliminated the need for blood and instead differentiated stem cells into immune cells, or natural killer cells, and then genetically engineered those.”
Matosevic said novel Purdue immunotherapy can be considered to have a true off-the-shelf source.
“We can envision having unlimited supplies of these stem cells ready to be engineered,” Matosevic said. “This does not require blood to be sourced. And because these are human cells, they are directly usable in human patients.”
Validation and next development steps
The research team tested its treatment by conducting animal studies with mice bearing human brain tumors, which were treated by direct injection of the newly engineered immune cells.
“Our preclinical studies showed these immune cells to be particularly remarkable in targeting and completely eliminating the growth of the tumors,” Matosevic said. “We found that we can engineer these cells at doses suitable for clinical use in humans. This is significant because one of the major hurdles to clinical translation of cell-based therapies to humans has been the poor expansion and lack of potency of cells that were sourced directly from patients. Using an off-the-shelf, fully synthetic approach breaks down significant barriers to the manufacturing of these cells.”
Matosevic said the next step to develop the glioblastoma treatment is to conduct clinical trials to treat patients with brain tumors, including those that were not successfully eliminated by surgery.
“Our ultimate goal is to bring this therapy to patients with brain tumors,” Matosevic said. “These patients urgently deserve better, and more effective, treatment options. We believe there is true potential for this therapy, and we have the motivation and capacity to bring it to the clinic.
“We are working with neurosurgical clinician collaborators to not only obtain funding, but also initiate clinical protocols,” he added. “We are also open to and always seeking new collaborations and partnerships with those who have interest in supporting our mission to translate this therapy to the clinic, where it is needed the most.”
Matosevic disclosed the innovative glioblastoma treatment to the Purdue Innovates Office of Technology Commercialization, which has applied for a patent from the U.S. Patent and Trademark Office to protect the intellectual property. Inquiries about the status of the intellectual property may be directed to Joe Kasper, assistant director of business development and licensing — life sciences, at [email protected].
Matosevic and the research team received funding from the National Institutes of Health, the V Foundation for Cancer Research, the Purdue Institute for Cancer Research and industry partners.