Innoblative Designs announced that it has initiated its first-in-human clinical experience, successfully treating a 64-year-old patient with stage II luminal A breast cancer. The initial lumpectomy case was performed by Dr. Cem Yilmaz, Breast Surgeon and Founding Director of the Istanbul Oncology Hospital in Istanbul, Turkey.
Breast cancer is a significant global health concern with documented impact in all countries. Once diagnosed, patients are faced with difficult decisions regarding available treatment options. Breast conservation therapy (BCT), which includes breast conserving surgery (BCS) plus radiation therapy, may be recommended. However, BCS is associated with a high rate of re-operation with nearly one-in-five patients requiring a follow up procedure within weeks of the initial excision to address residual cancer. In addition, subsequent radiation therapy can be cumbersome with frequent treatments over the course of several weeks or months and the potential for a range of unpleasant side effects. Many patients opt for a more drastic approach and undergo a mastectomy to avoid these concerns and alleviate fears of future reoccurrence.
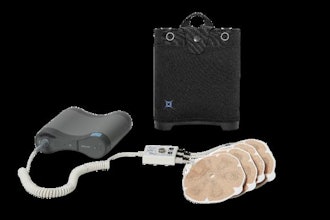
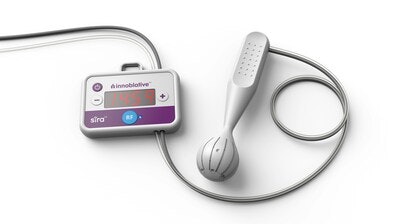
Innoblative's SIRA device aims to overcome these challenges and offer a better treatment option for BCS candidates. Its single-use, disposable applicator is designed specifically for intraoperative ablation of soft tissue, including potential residual cancer post-lumpectomy. With a unique spherical shape, the SIRA electrode circumferentially delivers radiofrequency (RF) energy to the entire cavity and yields reproducible ablation depths to provide greater confidence of a consistent thermal effect. Radiofrequency (RF) ablation has been shown in multiple long-term clinical studies to reduce reoperations and may reduce local recurrence in breast cancer treatment. However, conventional RF devices are not optimized to treat lumpectomy cavities and can lead to variable treatment depths and incomplete ablations.