Johnson & Johnson said the FDA granted Priority Review to the New Drug Application (NDA) filed for TAR-200, the company's intravesical gemcitabine releasing system for the treatment of patients with Bacillus Calmette-Guérin (BCG)-unresponsive high-risk non-muscle invasive bladder cancer (HR-NMIBC) with carcinoma in situ (CIS), with or without papillary tumors.
"TAR-200 represents an innovation in drug delivery that has not been seen in decades," said Yusri Elsayed, M.D., M.H.Sc., Ph.D., Global Therapeutic Area Head, Oncology, Johnson & Johnson Innovative Medicine. "The FDA Priority Review for TAR-200 underscores our mission to fundamentally change the way urologists treat certain types of bladder cancer."
The regulatory submission is supported by data from the Phase 2b SunRISe-1 study, which demonstrated an 82.4 percent complete response (CR) rate with 52.9 percent of patients remaining cancer-free at least one year or more after achievement of a CR (95 percent confidence interval [CI], 72.6-89.8). The majority of adverse reactions were mild and moderate. The most common adverse reactions (≥10 percent) included pollakiuria, dysuria, urinary tract infection, micturition urgency, hematuria, cystitis noninfective, and urinary tract pain. No systemic adverse reactions were reported. The findings were presented during a plenary session at the April 2025 American Urological Association Annual Meeting.
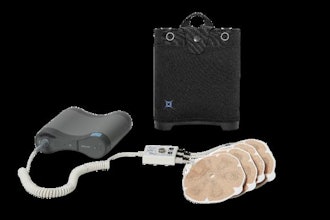
Despite advancements, there has been little change in the standard of care for patients with HR-NMIBC for over 40 years, and patients have limited treatment options if initial BCG therapy does not work. TAR-200 is the first and only intravesical drug releasing system (iDRS) designed to provide sustained local delivery of a cancer treatment into the bladder. TAR-200 remains in the bladder for three weeks per treatment cycle. A healthcare professional places it into the bladder using a co-packaged urinary placement catheter in an outpatient setting in less than five minutes. There is no need for general anesthesia, further monitoring, or other restrictions immediately post-insertion within the healthcare provider's office.