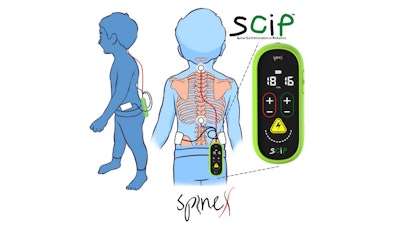
SpineX, a clinical stage medtech company, today announced the results its first in human study in children with cerebral palsy.
The study is published in the medical journal Nature Communications demonstrating functional improvements with its proprietary non-surgical treatment SCiP (SpinalCord Innovation in Pediatrics) in children with Cerebral Palsy (CP).
“A pilot study combining non-invasive spinal neuromodulation and activity-based neurorehabilitation therapy in children with cerebral palsy”, led by Dr. Susan Hastings, PT, DPT and Dr. V Reggie Edgerton, PhD is the result of years of work championed by the two stalwarts of their respective fields. This study discusses how delivering non-invasive spinal neuromodulation, using SCiP, during physical therapy improved voluntary sensorimotor function in 16 out of 16 children over a wide range of ages and severities of CP.
SpineX was awarded the Breakthrough Device Designation (BDD) from U.S. FDA for SCiP and the proposed treatment of CP. In addition, SpineX has engaged with the FDA to gain alignment on a proposed clinical trial to be conducted in 2023; the results of which are anticipated to lead to FDA clearance of the SCiP device for the treatment of CP.






















