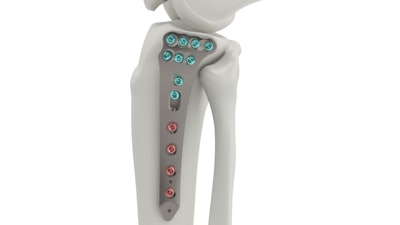
Tyber Medical, an orthopedic device manufacturer providing private label orthopedic implants for the trauma, extremity, and spine markets, has received FDA approval for its Proximal Tibia Plating System. The Tyber Medical System consists of two indication categories: Complete Articular and Partial Articular Fractures. Both designs stabilize bone fragments to facilitate highly focused and efficient healing of a wide array of injuries to the tibia, fibula, and femur.
"This innovative addition to the Tyber Medical portfolio aligns to meet the needs of our customers by offering multiple solutions to challenging anatomy," said David Hannah, Chief Technology Officer at Tyber Medical. "This comprehensive system offers anatomic plating to support a wide range of trauma needs."
The Tyber Medical Proximal Tibial Plating System is engineered to accommodate surgeon preferences, increase procedural efficiency, and enhance plate fit and screw placement. The Proximal Tibia system is anatomically designed to address a variety of indications and includes variable angle locking and non-locking screws.






















