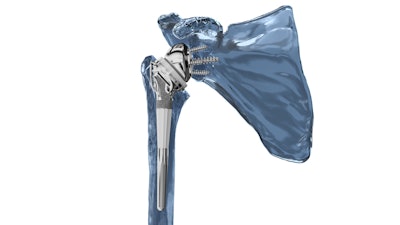
Catalyst OrthoScience has received 510(k) clearance from the FDA and the first surgeries have been successfully performed for the Catalyst Fracture Shoulder System, a reverse for fracture arthroplasty system to treat proximal humeral fractures (PHFs).
“The use of reverse shoulder arthroplasty (RSA) for the treatment of PHFs is steadily increasing,” said Dr. Steven Goldberg, chief medical officer of Catalyst. “One of the most critical challenges in treating PHFs is ensuring tuberosity fixation and healing. Traditional reverse implants are designed to treat shoulder arthritis, but fractures are a completely different problem with different objectives for the surgeon. Catalyst has engineered a solution that specifically addresses the complexities of PHFs in an efficient and novel way.”
One of the first cases using the new fracture system was successfully performed by Dr. Matthew D. Budge from Salem, Oregon.
“The unique design of the proximal body greatly simplified the usual difficulties encountered with tuberosity management. In addition, the ability to get excellent diaphyseal fixation of the implant without cement or screws greatly improved the efficiency of the case. Overall, I was very impressed," Budge said.
Key elements of the Catalyst Fracture System for RSA include:
- Tuberosity-specific fossa designed to maximize tuberosity contact and healing to a porous surface.
- Patented tuberosity retention rails designed to further restrict tuberosity motion post-operatively.
- Uncemented, secure press-fit diaphyseal fixation and strong rotational stability, with cemented options as well.
- Compatibility with glenospheres and baseplates in standard, augmented, and lateralized configurations to suit nearly every type of glenoid anatomy.
Catalyst will complete a limited user release to gather further data on the system’s performance ahead of a broader commercial release in 2025.






















