GT Medical Technologies, a medical device company focused on improving treatment for patients with brain tumors, has completed its oversubscribed $53 million Series D equity financing.
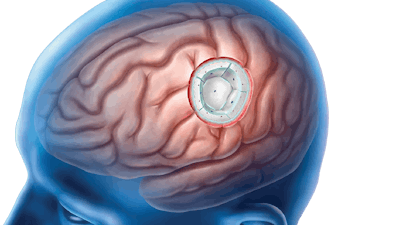
The company will use the funds to drive the expansion of its U.S. commercial activities for GammaTile, a form of radiation therapy placed at the time of brain tumor removal surgery, delivering immediate, targeted radiation to the tumor site when cancer cells are at their lowest levels.
GT said that unlike conventional approaches, which often require a delay between surgery and radiation therapy to allow for wound healing, GammaTile eliminates the treatment gap. By delivering immediate, localized radiation directly at the tumor site, it is designed to maximize the treatment's effectiveness against remaining cancer cells to reduce the risk of regrowth.
The new funding will also be used to complete enrollment of GT's ROADS randomized controlled trial for newly diagnosed brain metastases and commence an additional randomized control trial of GammaTile in newly diagnosed glioblastoma cases.