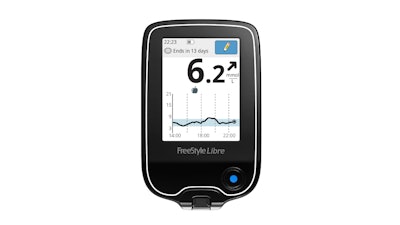
The FDA has issued a Class I recall for Abbott's FreeStyle Libre glucose monitors over concerns that the rechargeable lithium-ion batteries may get extremely hot, spark, or catch on fire if not properly stored.
The recall impacts approximately 4.2 million devices distributed in the U.S. between November 2017 and February 2023.
The FDA said the potential for overheating, spark or fire may occur when charging the Reader with non-Abbott adapters or non-Abbott USB cables along with misuse of the Reader and its components. Examples of misuse include exposure to liquids, damage, and introduction of foreign material into the ports.
The Abbott-provided USB cable and power adapter limit the current to safely charge the battery, whereas USB cables and power adapters manufactured by a third party may allow much higher power, increasing the risk of fire.
The Reader, if not properly stored, charged, or used with its Abbott provided USB cable and power adapter, may expose users to extreme heat and/or fire which can cause serious injuries or death. Additionally, users may delay or miss a critical diabetes treatment if the system cannot be used after is damaged by extreme heat.
Abbott reported 206 incidents, including at least seven fires, one injury, and no deaths involving this issue.