Neurolief, a medical neurotechnology innovator focused on neurological and neuropsychiatric disorders, today announced positive results from a planned interim analysis of the ongoing randomized controlled MOOD pivotal trial. MOOD is studying RelivionDP, a new novel neuromodulation therapy for treatment of Major Depressive Disorder (MDD).
At a meeting of the study's independent Data Monitoring Committee (DMC), statistical results from a pre-planned efficacy and safety interim analysis were reviewed. Based on data from 80% of the originally planned study sample size, the DMC issued a recommendation to continue patient enrollment up to the final planned sample size, as the interim results are "positively favorable". Full study results will be announced and submitted to the FDA and CE after the last patient completes the treatment protocol, expected in the 2nd quarter of 2024.
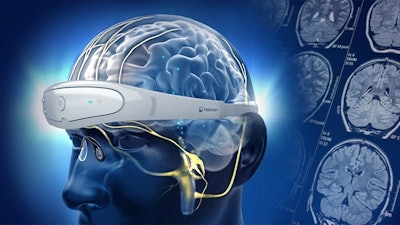
The RelivionDP is a neuromodulation system designed for treatment of depression and was awarded the FDA's Breakthrough Device Designation for its novel technology. Similar to a headset, the patient places the device on their head to administer treatments. Utilizing three adaptive output channels, the device transfers mild electrical pulses to the brainstem via six branches of the occipital and trigeminal nerves. This mechanism stimulates the release of neurotransmitters in the brainstem and modulates brain networks associated with mood. Part of a digital therapeutics platform, RelivionDP uses a dedicated smartphone app and a cloud database to allow psychiatrists to remotely monitor patients, analyze their data, and personalize treatments to enhance outcomes.
The MOOD clinical trial, conducted in 12 clinical sites across the US and 1 in Israel, is a prospective, multi-center, placebo controlled, randomized double-blind clinical trial. The study's primary endpoint assesses changes in depression symptoms from baseline to 8 weeks post-treatment initiation when using RelivionDP compared to the control group, in patients suffering from Major Depressive Disorder who failed to achieve satisfactory improvement from previous antidepressant medications.






















