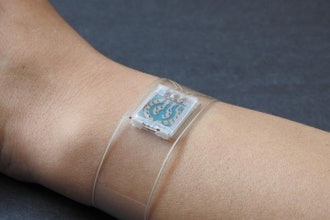
Medtronic today announced CE (Conformité Européenne) Mark approval for its new all-in-one, disposable Simplera continuous glucose monitor (CGM) featuring a two-step insertion process. The company's newest no-fingerstick sensor does not require over tape and is integrated with the InPen smart insulin pen, which provides real-time, personalized dosing guidance to help simplify diabetes management.
"Patients with diabetes can get overwhelmed by the sheer number of decisions they need to make on a daily basis. As a physician, I appreciate the ability to introduce this solution by Medtronic as it provides real-time, personalized guidance to help patients stay in range. For instance, when it detects someone is consuming a meal and their glucose levels are trending high, it alerts the person to help make diabetes management easier and provides peace of mind," said Dr. Sandra Schlüter, Endocrinologist, Germany, Head of AGDF.
"Despite the rapid adoption of CGM over the past decade, less than 30% of individuals on MDI therapy using a CGM achieve glycemic targets — highlighting a significant unmet need. We're excited to help more people to reach their goals with our advanced algorithm in InPen™ powered by our smallest and most comfortable CGM to-date," said Que Dallara, EVP and President, Medtronic Diabetes. "This newest addition of a Smart MDI solution to our holistic portfolio demonstrates our commitment to meeting people where they are in their diabetes journey with simplified solutions that help make life with diabetes easier."
Simplera is indicated for ages 2+ and compatible with iOS and Android. Simplera is not approved by the FDA and is limited to investigational use in the U.S. Medtronic's automated insulin delivery (AID) system integrated with this next-generation sensor is currently under review for CE Mark and is not commercially available in the U.S. or in Europe.