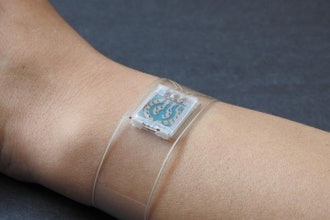
Vibrato Medical announced that data from an early feasibility study of Non-Invasive Therapeutic Ultrasound (TUS) to treat Chronic Limb-Threatening Ischemia (CLTI) has successfully met its endpoint. The study evaluated patients with infrapopliteal PAD and measured changes in foot perfusion and oxygenation as well as therapy tolerance, compliance and perception.
The data from the 12-patient Prelude study were presented by Mahmood Razavi, MD, Director of Clinical Trials and Research Center at the Vascular & Interventional Specialists of Orange County, as a podium presentation during the annual VIVA conference at Wynn Las Vegas on October 31, 2023.
Patients in the trial had Rutherford class 3, 4 or 5 PAD, meaning severe claudication, ischemic rest pain or tissue loss including nonhealing ulcers. The trial participants received 30-40 TUS treatment sessions over two months. At the end of the treatment:
- Each individual patient demonstrated a statistically significant improvement in perfusion
- Toe perfusion increased acutely by 180 ± 34 percent (p<0.001)
- Tissue oxygenation increased by +17 percent (p=.02)
- Patients showed excellent therapy tolerance, compliance and feedback
Over 18 million people in the United States have PAD, a disease that occurs when the peripheral arteries narrow, making it more challenging to carry blood away from the heart to other parts of the body. It is estimated that two million people with PAD have blockages so extreme they have advanced to CLTI, a chronic condition that can cause extreme pain, sores and wounds that do not heal and can result in the amputation of the affected limb. The unmet clinical needs for this population are extremely high.
Vibrato’s technology is a wearable therapeutic ultrasound device designed to promote vasodilation and vessel growth.