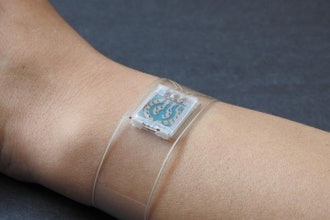
Xstim, a developer and manufacturer of bone growth stimulation systems, said it received Premarket Application (PMA) approval from the FDA for Xstim Spine Fusion Stimulator. The Xstim Spine Fusion Stimulator represents a milestone in the company's product lineup of bone growth stimulators. The capacitively coupled device emits a low-energy signal that promotes bone healing after spinal fusion surgery. Commercial availability of the Xstim device is slated for the second quarter of this year, with a targeted and phased launch plan in place to ensure widespread accessibility.
The Xstim device has been designed to prioritize patient ease of use and wearability, offering a noninvasive alternative for indicated cases.
"Xstim, Inc. is dedicated to empowering patients and surgeons to elevate the quality of care in spinal fusion rehabilitation. The introduction of the Xstim Spine Fusion Stimulator represents the beginning of our robust pipeline of bone growth stimulation innovations. We are poised to collaborate with healthcare providers and distribution partners to enhance bone fusion outcomes and elevate patient satisfaction. This FDA approval underscores our leadership in and dedication to the bone growth stimulation market," said Xstim CEO Jeremy Perkins.