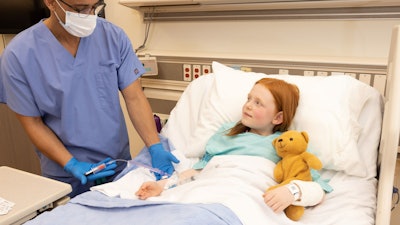
BD today launched new needle-free blood draw technology compatible with integrated catheters, helping to further enable the company's vision of a "One-Stick Hospital Stay."
With 510(k) clearance from the U.S. Food and Drug Administration (FDA), the new PIVO Pro Needle-free Blood Collection Device features design improvements to achieve the first and only compatibility with integrated and long peripheral IV catheters, including the new Nexiva Closed IV Catheter System with NearPort IV Access. This expands on current PIVO compatibility with traditional short peripheral IV catheters available since 2017.
The new solution combines the clinical benefits shown for the integrated closed Nexiva Catheter System, including longer dwell times and reduced catheter complications, with the ability to draw high-quality blood samples directly from a patient's peripheral IV line with PIVO Pro, reducing the need for additional needlesticks. With needle phobia experienced by more than 60 percent of the adult population, this solution helps improve the patient experience by alleviating fear and anxiety associated with repetitive needlesticks.
PIVO Pro combined with Nexiva with NearPort IV Access is designed to access optimal blood draw conditions and help improve clinician efficiency and patient experience. The innovative line draw solution has been shown to help optimize IV performance, reduce sample errors that can result in redraws and delays in patient care while reducing complications that lead to unnecessary procedures and IV replacements. By removing the needle from blood draws and reducing IV replacements, it may reduce the risk of needlestick injury and blood exposure for clinicians while preserving a patient's vessel health.






















