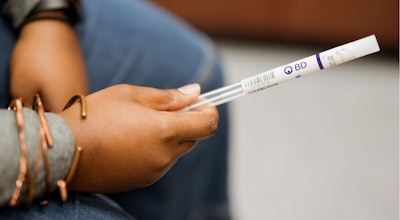
BD has submitted an application to the FDA for a new, at-home human papillomavirus (HPV) test that enables patients to self-collect a sample using a swab the size of a Q-tip that simplifies processing at the lab.
When available, the new HPV test from BD will include a self-collection swab technology that offers a safe, simple and non-invasive collection experience without the need for liquids or complex devices. The stability of the swab also allows for convenient mailing from home to the lab, removing logistical barriers and supporting broader participation in cervical cancer screening programs.
At the lab, the self-collected swab needs no manual sample preparation by clinical laboratory technologists, which allows them to focus on higher value work. The sample is simply placed into the BD COR System and the fully automated process uses sophisticated robotics to prepare, analyze and report results for each sample. Using an internal cellular control and minimizing manual touch and intervention ensures the integrity of the specimen from collection to delivery of dependable, high-quality results.
The BD Onclarity Assay can also report more individual high-risk strains of HPV than any other test available today. This is a must-have attribute in today's testing paradigm, due to the shifting prevalence of high-risk genotypes beyond HPV 16/18.