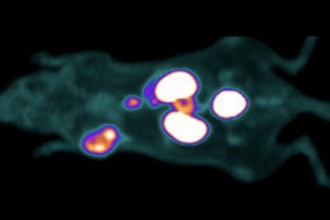
Median Technologies is expanding its portfolio of services with Imaging Lab, a new entity whose mission is to use AI, data mining and radiomics technologies to exploit imaging data from clinical trials in oncology.
The creation of Imaging Lab covers iCRO's activities for image management in the development of new oncologic drugs and iBiopsy's activities for the development of software as medical device targeting early diagnosis of cancers, especially lung cancer.
"We are seeing a paradigm shift of pharmaceutical companies towards new drug candidates targeting patients with early-stage cancers," said Fredrik Brag, CEO and founder of Median Technologies. "The synergy between our iCRO and iBiopsy businesses is perfect to respond to this change: iBiopsy develops software as medical device, integrating AI technologies, which allow the diagnosis of diseases at a very early stage, when patients are still asymptomatic. At the same time, iCRO has extensive knowledge of image processing and its management in clinical trials. The cross-fertilization of our two businesses will enable us to leverage imaging data in conjunction with other clinical information in an unparalleled way and provide biopharmaceutical companies with tools for Go/No-Go decisions in trials," adds Fredrik Brag.
Imaging Lab will provide new answers in four areas that determine the success of clinical trials: selection of patients included in trials, especially inclusion of patients diagnosed at early stages of disease thanks to AI technologies, prediction of response to therapy, measurement of disease progression, and evaluation of the safety of drug candidates. The goal is to optimize development plans, including facilitating Go/No-Go decisions to increase the success rate of clinical trials. This rate is especially low in oncology, generating an average development cost of $2.8 billion to take a new molecule to market, compared with an average of $1 billion per new molecule brought to market for other therapeutic areas.
"Our experience of image management in clinical trials has shown that trial data is vastly underutilized. We can extract much more information from images through the widescale use of data mining, AI, and radiomics and use these technologies to better support our customers and biopharmaceutical partners in their clinical developments," said Nicolas Dano, COO iCRO of Median Technologies.