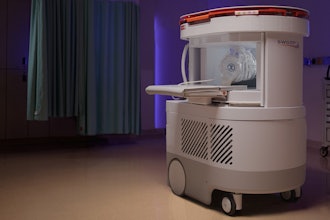
EMVision, an Australian medical device company, has unveiled its first responder proof of concept device, designed for deployment via road and air ambulances.
The backpack-sized scanner weighs less than 25 pounds and will enable paramedics to scan and send images to stroke experts from the site of a suspected stroke.
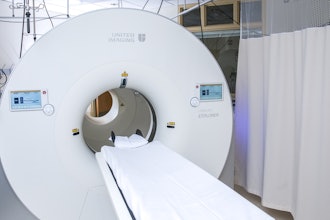
The first responder device leverages the underlying innovation from EMVision’s emu brain scanner. The emu is a trolley-mounted device, targeted for use in intensive care units, stroke and neurology wards, and rural emergency departments, involving a simple cap placed on the patient's head for quick scans and timely stroke and stroke subtype assessment. It couples safe, ultra-high frequency radio signals with powerful artificial intelligence to produce rapid insights at the patient’s bedside.
It's a second-generation device with a lighter and miniaturized design, with expanded antenna coverage designed to offer full brain coverage in a single scan. It will now be the subject of a series of studies and developments to ensure its usability, reliability, and functionality to support the regulatory approval pathway. Both devices can be operated at the point-of-care by any healthcare professional with minimal training.
The emu and the first responder device are not available for sale nor evaluated by the FDA or other global regulators. The devices may only be used by authorized sites in controlled clinical investigations. Market entry for the emu device is planned for next year, with the first responder device to follow.