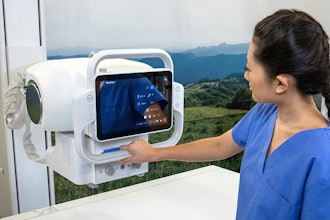
SamanTree Medical today announced that it has received FDA 510(k) clearance of its Histolog Scanner for imaging of the internal microstructure of tissues including, but not limited to, the identification of cells, vessels and their organization or architecture. This device is designed to provide real-time, high-resolution imaging of fresh tissue surfaces, offering surgeons and pathologists this information for excised tissue.
The Histolog Scanner enables confocal microscopy, allowing for the immediate imaging of resected tissues. This advancement aims to enable physicians to improve their efficiency.
Prof. Dr. Ari D. Brooks, Director of Endocrine and Oncologic Surgery, Pennsylvania Hospital, Philadelphia, PA said “The Histolog technology has the potential to significantly reduce the cost, resources and delays associated with the current lack of real-time high-resolution imaging”. Prof Dr. Douglas Scherr, Clinical Director of Urologic Oncology at the Weill Medical College of Cornell University said “Providing imaging data at this resolution has the potential to aid in patient care. In our practice and across Urology, as a field, we are facing limitations in the ability to provide timely decisions to our patients. We will count on the Histolog Scanner to provide us with the ability to image the tissues’ microstructures in real-time and to enable us to make immediate decisions.”
With this clearance, SamanTree is set to accelerate its U.S. market entry, ensuring that more healthcare providers can leverage the benefits of this technology.