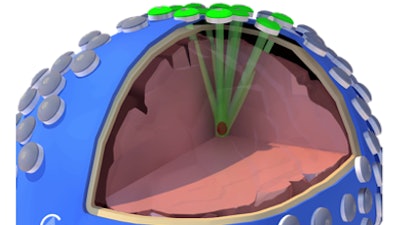
Cordance Medical, a medical device company focused on opening the Blood-Brain Barrier (BBB) to facilitate liquid biopsy, announces that its NeuroAccess device has been granted Breakthrough Device Designation by the U.S. Food and Drug Administration (FDA).
NeuroAccess is designed for adults (aged 22+) with known or suspected brain tumors, enabling trained healthcare professionals to non-invasively elevate cell-free DNA (cfDNA) analytes in blood circulation. This procedure, called SonoBiopsy, enhances existing oncology liquid biopsy tests.
The FDA's Breakthrough Device Designation is conferred upon technologies that offer superior treatment or diagnosis for life-threatening or debilitating conditions. This designation underscores the transformative promise of the NeuroAccess platform in addressing the unmet medical needs to expand the continuum of care for brain tumor patients by facilitating safe procedures to obtain molecular characterization of their tumors. Under the program, Cordance Medical will receive prioritized review and accelerated interaction with the FDA.
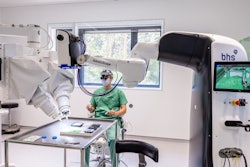
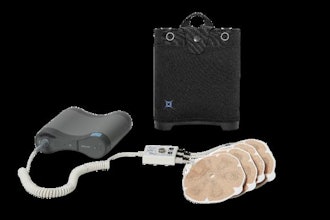
The NeuroAccess technology employs focused ultrasound in combination with microbubbles to open the BBB in a safe, temporary, and non-invasive manner. Designed to be portable, NeuroAccess aims to enable SonoBiopsy procedures broadly across community clinics and hospitals alike.
Numerous preclinical studies employing focused ultrasound and microbubble-mediated BBB disruption have shown potential benefits for a range of neurological disorders. This modality provides a targeted, transient opening of the BBB, unlocking the potential to revolutionize both liquid biopsy and drug delivery techniques in conditions like glioblastoma multiforme (GBM), brain metastasis, Alzheimer's, Parkinson's, and other neurologic diseases.