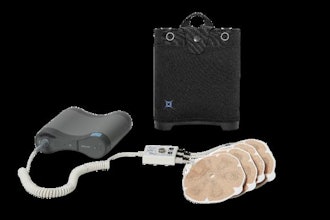
DermaSensor announces FDA clearance for its real-time, non-invasive skin cancer evaluation system. For the first time, the 300,000 primary care physicians in the U.S. can now be equipped to provide quantitative, point-of-care testing for all types of skin cancer. Better identifying skin cancer in a primary care setting is designed to accelerate patient access to necessary care.
Primary care physicians are at the forefront of healthcare delivery, handling diverse and increasing medical concerns while playing a critical role in the early identification of disease. To date, PCPs’ limited options for evaluating suspicious moles have been the naked eye or magnified visual examination of lesions, both of which are dependent on clinical training and subjective judgment. But now, physicians can use DermaSensor’s AI-powered spectroscopy technology to non-invasively evaluate cellular and subcellular characteristics of a lesion in question for skin cancer. The wireless, handheld device then provides an immediate, objective result using an FDA-cleared algorithm.
The FDA pivotal study of over 1,000 patients, led by the Mayo Clinic across 22 study centers to validate device performance, showed that the device had a sensitivity of 96 percent across all 224 skin cancers. A negative result had a 97 percent chance of being benign for all skin cancers. In a companion clinical utility study with 108 physicians, the DermaSensor device was found to decrease the number of missed skin cancers by half (from 18% to only 9%), increasing the physicians’ accuracy and confidence in assessing cancerous lesions.
The benefits are as much for dermatologists as they are for PCPs. DermaSensor is expected to improve primary care and dermatology collaboration, enabling better-prioritized referrals in addition to the referral of more patients with skin cancer. The company has conducted 13 clinical studies in the last decade, six of which provided the principal support for FDA clearance.