The first commercial patients in the U.S. have been successfully implanted with the FDA-approved WiSE System.
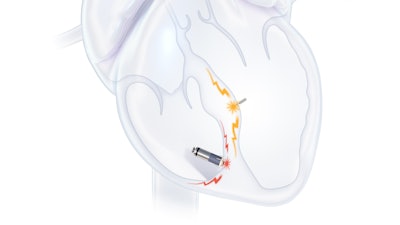
The WiSE System is one of the first leadless devices to deliver left ventricular endocardial pacing (LVEP)—offering a more physiological approach that mimics the heart's natural electrical activation by stimulating the endocardial tissue inside the left ventricle. This expands treatment options for heart failure patients who have exhausted conventional therapies.
The procedure took place at St. David's Medical Center of Austin in Texas, one of several leading institutions participating in the initial limited market release of the WiSE System.
Unlike conventional LV leads placed in the coronary sinus, the WiSE System delivers pacing via a miniaturized electrode implanted directly into the left ventricular endocardium and activated by ultrasound energy. This direct approach enables more flexible lead placement and expands access to those who were previously considered untreatable.
An additional implant was simultaneously completed by Dr. Niraj Varma at the Cleveland Clinic, another center participating in EBR's limited market release.
EBR will continue its phased rollout throughout 2025, partnering with select centers experienced in CRT and leadless pacing. A broader U.S. commercial launch is expected in 2026.