Samsung announced that the Irregular Heart Rhythm Notification (IHRN) feature of the Samsung Health Monitor app has received FDA clearance. Together with the app’s existing on-demand Electrocardiogram (ECG) function, the IHRN feature proactively monitors heart rhythms suggestive of atrial fibrillation (AFib), right from their wrist.
“We’re excited to announce that Irregular Heart Rhythm Notification, designed to help millions of people around the world who may not be aware of a potential heart risk, has been cleared by the FDA,” said Hon Pak, Vice President and Head of the Digital Health Team, MX Business at Samsung Electronics. “This is yet another example of how Samsung prioritizes proactive safety solutions and enables users to receive a more holistic understanding of their cardiovascular and overall health.”
Cardiovascular disease remains one of the world’s leading causes of death, and AFib — a type of arrhythmia — is widely considered a warning sign for major cardiovascular issues that can increase the risk of stroke, heart failure and other cardiovascular complications. Moreover, some of AFib cases are asymptomatic or even silent, leaving people unaware of their risk.
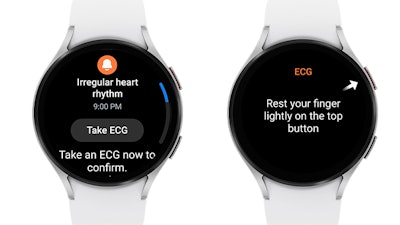
That’s why Galaxy Watch offers tools to help users better understand their heart health using the Samsung BioActive Sensor, including on-demand ECG recording and HR Alert function that detects abnormally high or low heart rates. The addition of the new IHRN feature enables Galaxy Watch users to monitor another aspect of their health.
Once activated in the Samsung Health Monitor app, the feature will check for irregular heart rhythms in the background via Galaxy Watch’s BioActive Sensor. If a certain number of consecutive measurements are irregular, Galaxy Watch warns the user of potential AFib activity, prompting them to take an ECG using their watch for a more accurate measurement.
The Irregular Heart Rhythm Notification feature will be available as part of the newly announced One UI 5 Watch, coming first to the upcoming Galaxy Watch devices later this year, and later expanding to previous editions.