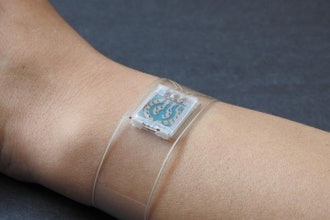
Element Science announced that its Jewel Patch Wearable Cardioverter Defibrillator (Patch-WCD) has received FDA approval of its Premarket Approval (PMA) application. This positive decision paves the way for the Jewel Patch-WCD to offer protection for U.S. patients who have an elevated temporary risk of sudden cardiac arrest. The Jewel Patch-WCD received the European Union’s CE mark certification and Great Britain’s UK Conformity Assessed (UKCA) marking in January 2024.
The Jewel Patch-WCD combines the principles of human-centered design with the latest advancements in machine learning. With features like water resistance that allows for continuous protection during showering, and a discreet profile, the Jewel Patch-WCD allows patients to live their lives as they want, without the constraints of traditional devices. Its algorithm and user-friendly design make it a reliable guardian patients can use in the comfort of their own homes and during their daily activities to protect against sudden cardiac arrest.
The FDA approval of Jewel Patch-WCD affirms its clinical safety and effectiveness and solidifies Element Science's leadership in wearable life-saving cardiovascular technology.