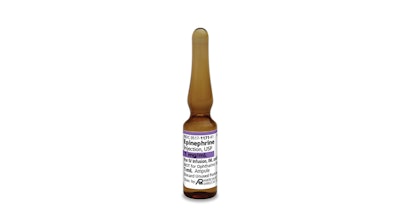
American Regent announces the launch of sulfite-free Epinephrine Injection, USP.
Epinephrine Injection, USP, is indicated for emergency treatment of allergic reactions (Type I), including anaphylaxis, which may result from insect stings or bites, foods, drugs, sera, diagnostic testing substances and other allergens, as well as idiopathic anaphylaxis or exercise-induced anaphylaxis. Epinephrine is also indicated to increase mean arterial blood pressure in adult patients with hypotension associated with septic shock.
"With the approval of this generic alternative we continue to show our commitment to ensuring both patients and providers have access to quality treatment options," stated Joann Gioia, Vice President and Chief Commercial Officer at American Regent, Inc.