Researchers at the University of Wisconsin–Madison, the first U.S. clinical evaluation site for GE Healthcare’s industry-first silicon-based photon counting CT, will begin human scanning using the device, which is engineered with Deep Silicon detectors with the goal of greatly enhancing imaging capabilities to help clinicians improve patient outcomes across oncology, cardiology, neurology, and other clinical CT applications.
The collaboration comes nearly one year after GE Healthcare announced its first clinical evaluation site at Karolinska Institute and MedTechLabs in Sweden. Since then, the company has made rapid progress in enhancing the developing technology, building a new system prototype to include:
- A larger detector with the possible goal of enabling quicker scan times as well as expanding coverage;
- ECG-gated cardiac scan capabilities designed for coronary artery imaging; and
- Faster acquisition speed with the intent to reduce the likelihood of blurred images due to motion.
“Photon counting CT has promise to embody the best of CT imaging available to date,” explains Dr. Meghan G Lubner, professor of radiology at the UW School of Medicine and Public Health. “This technology has the potential to expand the scope of current indications by combining refined energy resolved data, high spatial resolution, reduced noise and improved soft tissue contrast. We are working with GE Healthcare by testing their novel photon counting solutions in human subjects to assess issues ranging from improving commonly encountered CT image quality limitations to evaluating whether previously out of reach clinical questions can now feasibly be answered.”
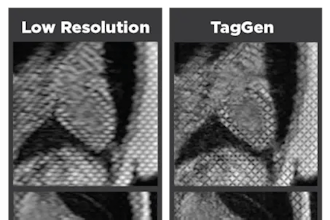
Photon counting CT could potentially advance the capabilities of CT, including the visualization of minute details of organ structures, improved tissue characterization, more accurate material density measurement (or quantification) and lower radiation dose.
GE Healthcare is pursuing a unique approach to photon counting CT, which may enable higher spatial and spectral resolution at the same time, thanks to several advantages provided by Deep Silicon detectors, including: the detector’s material purity, innovative geometric design, and true multi-bin technologies for high performance spectral imaging. As such, the research being done at UW–Madison will assist GE Healthcare in better understanding the heights of these unique capabilities.
UW–Madison will facilitate human subject research and produce technical feedback to test and advance GE Healthcare’s photon counting CT technology with Deep Silicon. The study will assess reconstruction methods, image presentation workflows, and clinical benefits for specific pathologies and disease types to determine how to best optimize photon counting CT with Deep Silicon detectors to enable better visualization and utilization.