Rehabilitation robotics company Harmonic Bionics announced today that the company has registered their flagship exoskeleton Harmony SHR with the FDA as a Class II 510(k)-exempt device. The company will be exhibiting the Harmony SHR system at the American Occupational Therapy Association (AOTA) Inspire 2023 Annual Conference taking place this week in Kansas City, MO.
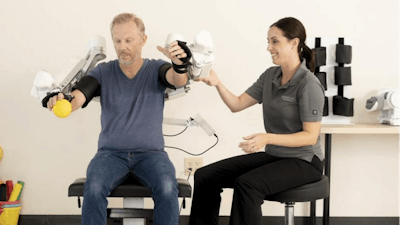
The Harmony SHR Robotic Rehabilitation System works with a patient's scapulohumeral rhythm (SHR) to enable natural, comprehensive therapy for both arms. As an upper extremity exercise device, Harmony may assist in the treatment of upper body movement impairments including neurological injury (such as stroke), neuromuscular disease/disorder, musculoskeletal disease, post-procedure musculoskeletal rehabilitation, and upper limb prosthetic or transplant rehabilitation.
"Our team has worked so hard to reach this milestone, but we are especially grateful to the therapists and clinicians we have worked with to ensure our Harmony SHR system is an effective tool for providers and their patients," said Harmonic Bionics CEO Christopher Prentice. "In addition to assisting in recovery, we know rehab robotics and technology is one way to address workforce challenges that are plaguing the healthcare industry and we believe Harmony can be a part of that solution."
Harmonic Bionics will be demonstrating the Harmony SHR system later this week in booth 126 at AOTA Inspire, the largest occupational therapy conference in the country. The company will also host a special seminar entitled "What is Bilateral Sync Therapy? Using Advanced Technology to Promote Neuroplasticity" presented by Harmonic Bionics' Clinical Education & Training Specialist on Friday, April 21 at 1:00 pm in Hall C, Theatre 2. Attendees will receive .75 contact hour (.75 NBCOT PDU).