VisAR, an augmented reality surgical navigation system from healthcare technology provider Novarad, has received FDA 510(k) approval for precision guided intraoperative spine surgery.
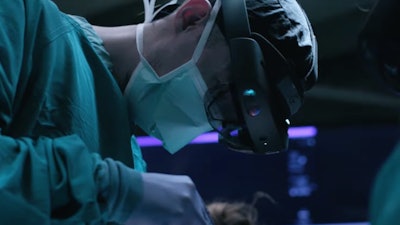
VisAR uses a patient's imaging data to create a 3-dimensional hologram that is visible through an optical visor and superimposed onto the patient with submillimeter accuracy. This allows the surgeon to focus directly on the surgical objective without looking away at a separate monitor.
"This is transformational technology that provides the precision of a robot, the portability of a stethoscope and the versatility of human powered intelligence," said Dr. Wendell Gibby, Novarad CEO and co-creator of VisAR. "Like a surgical GPS, VisAR provides a roadmap to guide the surgeon to the pathology of interest."
VisAR is an end-to-end solution with pre-surgical planning, virtual annotations, segmentation and bi-directional image connectivity. It features integrated 2D and 3D immersive navigation views with continuous hologram-to-patient registration. VisAR technology uses image visible CT fiducial markers for automatic registration. The operating room setup time is less than 2 minutes. Surgical accuracy is sub 2 mm for pedicle screw placement in both open and minimally invasive surgical (MIS) procedures.
Novarad has partnered with Microsoft to use off-the-shelf AR headset technology which allows for lowered cost and the ability to leverage expected hardware advancements. The untethered wirelessly connected Microsoft HoloLens 2 visor worn by the physician results in the smallest OR footprint of any system on the market. No other navigation equipment is required. VisAR is built on the Novarad imaging technology solution stack that provides interoperability, HIPAA compliance, image management, and deep security.
VisAR is currently available in the U.S., with usage being anticipated in other countries in the coming months. Head and Neck surgical approvals are currently in the consideration phase with the U.S. Federal Drug Administration.