TISSIUM, a medtech company specializing in biomorphic programmable polymers for tissue reconstruction, has earned FDA De Novo marketing authorization for COAPTIUM CONNECT with TISSIUM Light, an atraumatic sutureless solution for peripheral nerve repair.
This authorization represents a regulatory milestone for TISSIUM, further validating its biopolymer platform and enabling U.S. commercialization of its first product. COAPTIUM CONNECT is now the only FDA-authorized system designed for atraumatic sutureless nerve coaptation.
“This FDA marketing authorization validates over a decade of scientific and clinical commitment to developing next-generation solutions in tissue reconstruction. COAPTIUM CONNECT is the first demonstration of the transformative potential of our polymer platform and an important step in making atraumatic tissue repair available to patients," said Christophe Bancel, co-founder and CEO of TISSIUM.
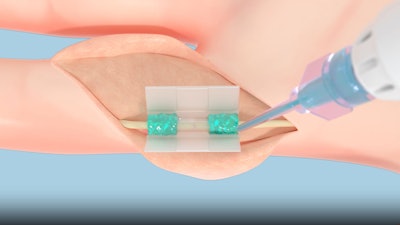
Peripheral nerve injuries affect hundreds of thousands of patients annually and are typically repaired using microsurgical sutures. However, this approach presents limitations—including technical complexity, risk of additional trauma, and variable outcomes. COAPTIUM CONNECT addresses these challenges by offering a reproducible, atraumatic sutureless alternative that preserves nerve integrity and simplifies the coaptation process.
In a recent clinical study on 12 patients with digital nerve injuries, COAPTIUM CONNECT achieved 100% procedural success, defined as successful atraumatic sutureless coaptation using the polymer-assisted coaptation device, with all patients regaining full flexion and extension of the injured digit and reporting no pain 12 months after the procedure.
TISSIUM plans to initiate commercial rollout of COAPTIUM CONNECT in the coming months. The COAPTIUM CONNECT System leverages TISSIUM’s unique biopolymer platform, invented by Maria Pereira, Jeffrey Karp and Robert Langer at the MIT and Brigham & Women’s Hospital, using its bioresorbable light-activated surgical polymer and its 3D-printed polymer chamber.
“This first product illustrates the technical versatility and the potential of the TISSIUM polymer platform, not only in peripheral nerve repair where other solutions are currently under development, but also in other surgical applications, such as atraumatic hernia repair and cardiovascular sealing," said Maria Pereira, co-founder and chief innovation officer.